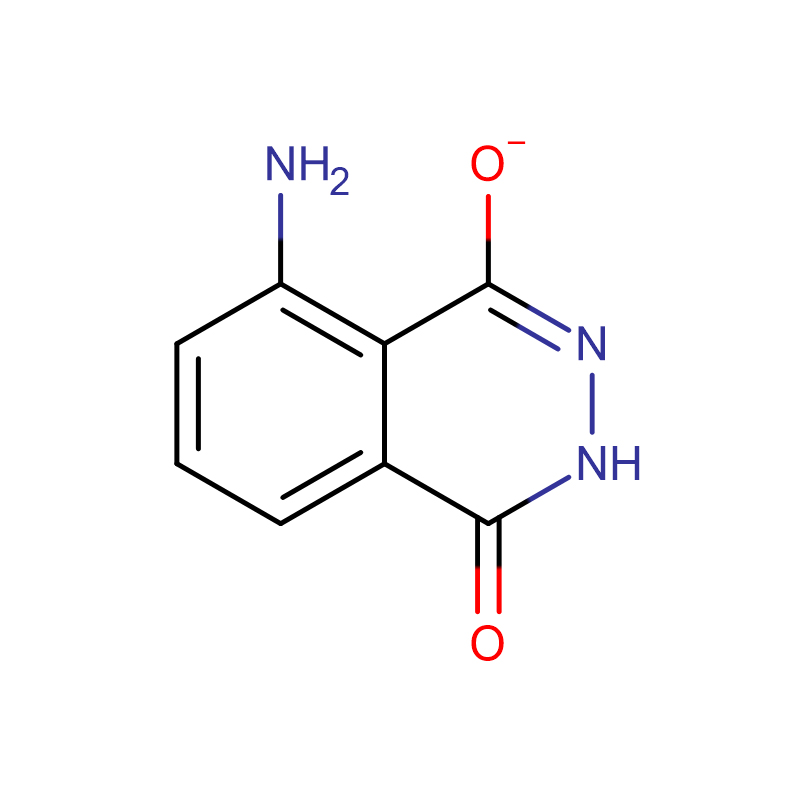
3-Aminophthalhydrazide Cas:521-31-3 98% Off-white to light yellow powder
| Catalog Number | XD90173 |
| Product Name | 3-Aminophthalhydrazide |
| CAS | 521-31-3 |
| Molecular Formula | C8H7N3O2 |
| Molecular Weight | 177.16 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 29339980 |
Product Specification
| Appearance | Off-white to light yellow powder |
| Assay | >98.0% min |
| Loss on Drying | <8.0% |
Chemifluorescence: Molecular luminol is a chemically fluorescent molecule that can be converted into excited state aminophthalic acid in the presence of hydrogen peroxide molecules, which emits strong fluorescence. Hydrogen peroxide is the product of many biooxidative reactions, so it is easy to link these biooxidative reactions with photodetection by introducing luminol. For example, the glucose oxidase/catalase probe can detect the concentration of hydrogen peroxide or glucose in the sample, and the response time is only 0.5s (dynamic method). Combining chemiluminescent substances with immunological reactions, and expressing the concentration of the detected immune components by light reactions, is called chemiluminescent immunoassay technology. In 1976, Shmeder proposed chemiluminescence immunoassay, using luminol-H202 and its derivative ABEI as the detection reaction display system. At present, luminol and its derivatives are the most commonly used markers in chemiluminescence immunoassay applications. Under alkaline conditions, catalyzed by microperoxidase, a large number of photons can be released. Luminol can be used for WesternBlot of HRP-labeled antibodies and nucleic acid hybridization detection of HRP-labeled probes. It is also used in modern criminal detective bloodstain detection. A compound that can be used as a chemiluminescent marker must meet the following conditions: the quantum yield of luminescence is high; its physicochemical properties match the system under study; its luminescent reaction is the result of the oxidation reaction of the luminescent substance; within the concentration range used Internally non-toxic to living organisms. Several types of chemiluminescence reagents are commonly used: acridine ester, a widely used chemiluminescence tracer, it is a tricyclic organic compound, easy to oxidize, and the oxidation reaction does not require a catalyst, and releases photons at 430nm; Luminol and isoluminol and their derivatives are the most mature chemiluminescent agents. As early as 1964, While reported on chemiluminescence, luminol, isoluminol and their derivatives were used for chemiluminescence. Immunoassays have achieved better results. Luminescence detection: the optimum fluorescence wavelength is 400nm (detection of chemiluminescence in 60mmK2S2O8, 100mmK2CO3, pH11.5 solution)
Chemiluminescence: As a sensitive assay method, chemiluminescence is widely used to detect free radicals and reaction metabolites produced in enzymes, cells and biological organisms. The light emitted by their oxidation products and metabolites can be used with various photometers. detection. Chemiluminescence is widely used in the screening and research of antioxidants, such as polyphenols, polysaccharides, flavonoids, and anthraquinones, because of its sensitivity, rapidity, simple operation and low price. The commonly used chemiluminescence systems for the determination of superoxide anions include xanthine oxidase-luminol, pyrogallol-luminol and dimethyl sulfoxide-luminol. The former is an enzymatic system, and the latter two It is a non-enzymatic system. The chemiluminescence systems for the determination of hydroxyl radicals mainly include copper sulfate-yeast (or bone marrow cells)-ascorbic acid-hydrogen peroxide, CuCl-H2O2-o-phenanthroline-carbonate buffer, copper sulfate-o-phenanthroline-ascorbic acid-hydrogen peroxide 5 chemiluminescence systems, ferrous sulfate-luminol-hydrogen peroxide and ferrous sulfate-luminol, all involve the classic Fenton reaction to generate hydroxyl radicals, and then attack the luminescent agent to generate chemiluminescence, which can be used to measure the removal of extracts The activity of reactive oxygen radicals. Experimental method: use pyrogallol-luminol chemiluminescence system: luminol is made into 0.05mol/L concentration solution with 0.05mol/L NaOH solution, stored in a dark place, and double distilled water before use Dilute to 1mmol/L solution, use 1mmol/L HCl to make 0.01mol/L solution of pyrogallol, store it in a refrigerator at 4°C, and dilute it with double distilled water to 16 times 6.25×10-4mol/L solution before use . 0.05mol/L pH10.2Na2CO3-NaHCO3 buffer (containing 0.1mmol/LEDTA) was prepared before use, and mixed with 1mmol/L luminol at 2:1 (volume fraction) to form luminol and carbonate buffer before the experiment mixture. During the measurement, inject 10.0 μL samples of different concentrations (0, 0.08, 0.4, 2 and 10 mg/mL) into the luminescent cell (with the sample buffer as the control), and then inject 6.25×10-4mol/L pyrogallol 0.05 Finally, 0.94 mL of a mixture of luminol and carbonic acid buffer was added to start the reaction (30°C), the luminescence intensity was counted at intervals of 2 s, and the total integrated luminescence intensity at 300 s was measured. The background luminescence intensity was the luminescence when pyrogallol was not added. value. In addition, there are chemiluminescence, fluorescence spectroscopy and NBS-dichlorofluorescein chemiluminescence system for the determination of fluorescence chemiluminescence, etc., their greatest advantage is high sensitivity.
Chemical Properties: Yellow crystalline powder. Easily soluble in lye, soluble in dilute acid, almost insoluble in water, and insoluble in alcohol. Neutral or slightly acidic solutions exhibit strong bright blue fluorescence when exposed to ultraviolet light. Melting point 329-332℃
Uses: As a chemical analysis reagent and indicator. Use Chemiluminescence analysis detection reagent (such as determination of metal cations or blood)
Uses: For chemiluminescence analysis, such as: metal cations, blood and glucocorticoids
Uses: Luminescence test: Emmax440nm (chemiluminescence; 60mmK2S2O8, 100mmK2CO3, pH11.5; after adding H2O2) chemiluminescence reagent and indicator, commonly used in chemiluminescence analysis, such as metal cations, blood immunity, etc.



![1,2,3,4-Tetrahydrobenzo[h]quinolin-3-ol CAS:5423-67-6 Off-white powder](https://cdn.globalso.com/xdbiochems/5423-67-6.jpg)