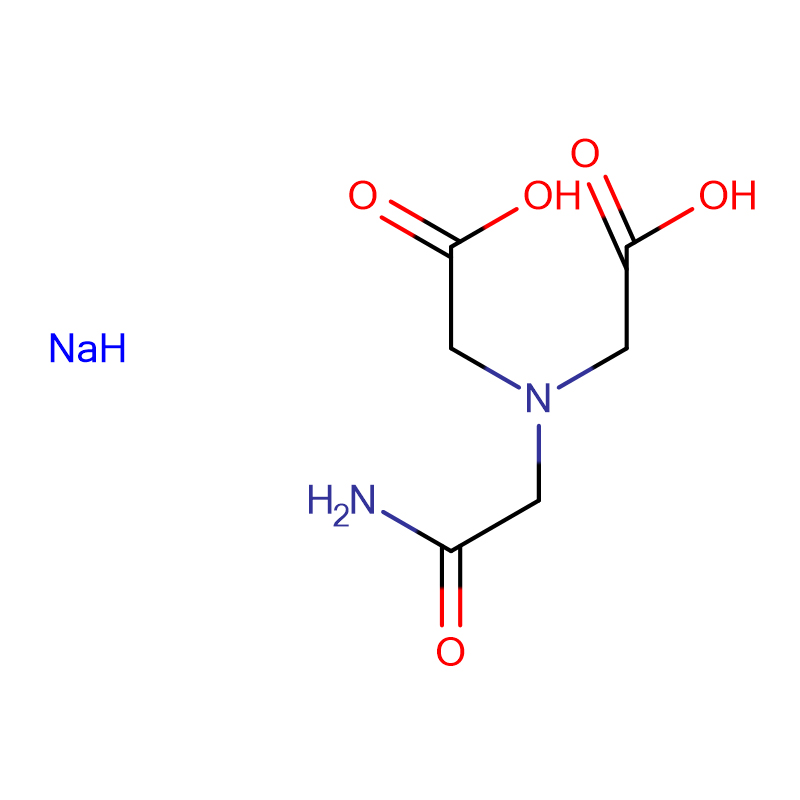
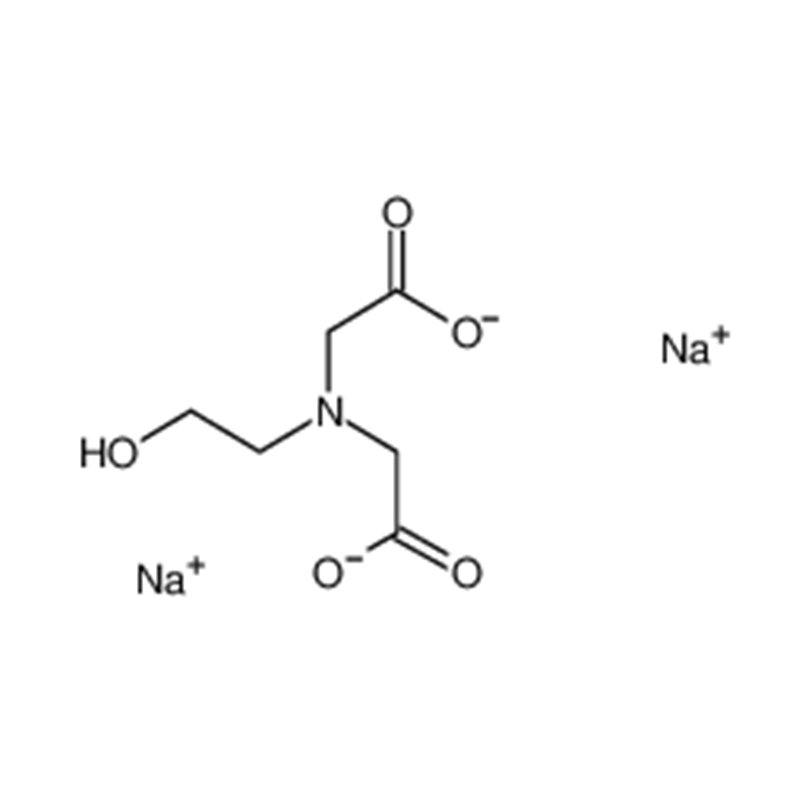
ADA DISODIUM SALT Cas:41689-31-0 N- (2- Amino- 2- oxoethyl)- N- (carboxymethyl) glycinedisodiumsalt White crystalline powder 98%
| Catalog Number | XD90092 |
| Product Name | ADA DISODIUM SALT |
| CAS | 41689-31-0 |
| Molecular Formula | H2NCOCH2N(CH2CO2Na)2 |
| Molecular Weight | 234.12 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 292419009 |
Product Specification
| Appearance | White crystalline powder |
| Assay | >98.0% |
| Storage Temp | Store at RT |
Buffer is a solution that can resist pH changes when a small amount of acid or alkali and water are added. The pH buffer system plays an important role in maintaining the normal pH value of organisms and the normal physiological environment. Most cells can only operate in a very narrow pH range. It needs to have a buffer system to resist the pH changes that occur during metabolism. There are three main pH buffer systems in the organism, they are protein and bicarbonate buffer systems. The amount of each buffer system It is different in various types of cells and organs.
The buffering effect of the buffer solution composed of the weak acid HA and its salt NaA on the acid is due to the presence of a sufficient amount of alkali A- in the solution. When a certain amount of strong acid is added to this solution, H ions are essentially consumed by A- ions:
Therefore, the pH value of the solution is almost unchanged; when a certain amount of strong base is added, the weak acid HA in the solution consumes OH- ions and hinders the change of pH.
Add a small amount of strong acid or strong base to the buffer solution, the pH value of the solution will not change much, but if the amount of acid or alkali is added, the buffer solution will lose its buffering effect. This shows that its buffer capacity has a certain limit.
The buffering capacity of a buffer solution is related to the concentration of the components that make up the buffer solution. The buffer solution composed of 0.1mol·L-1HAc and 0.1mol·L-1NaAc has a greater buffering capacity than the buffer solution of 0.01mol·L-1HAc and 0.01mol·L-1NaAc. This can be confirmed by calculation. However, the concentration of the components of the buffer solution should not be too large, otherwise, the interaction between the ions cannot be ignored.