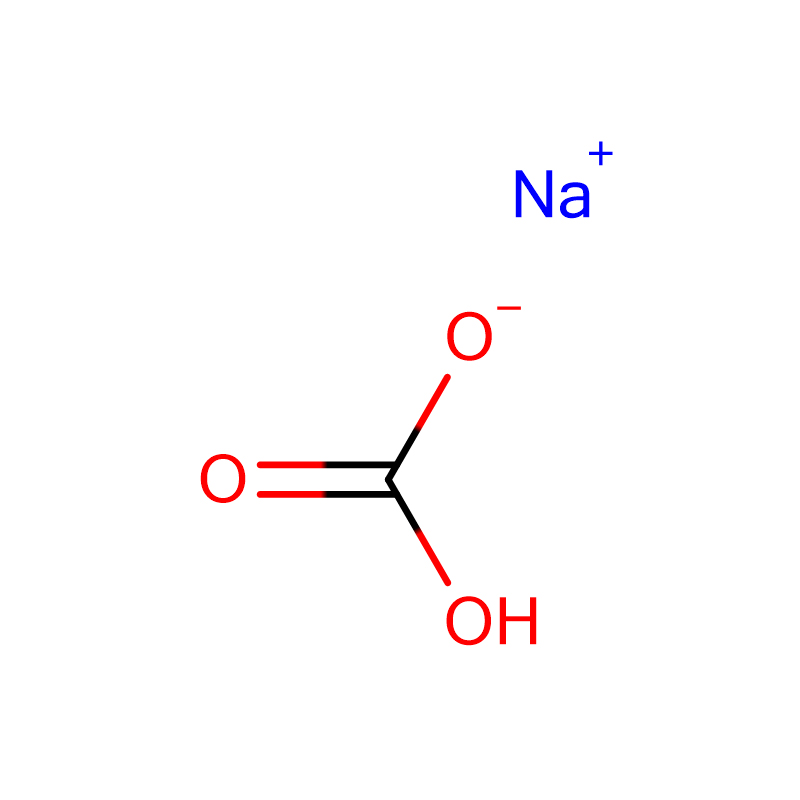
Sodium Bicarbonate Cas: 144-55-8
| Catalog Number | XD91855 |
| Product Name | Sodium Bicarbonate |
| CAS | 144-55-8 |
| Molecular Formula | CHNaO3 |
| Molecular Weight | 84.01 |
| Storage Details | 2-8°C |
| Harmonized Tariff Code | 28363000 |
Product Specification
| Appearance | White powder |
| Assay | 99% min |
| Melting point | >300 °C(lit.) |
| Boiling point | 851°C |
| density | 2.16 g/mL at 25 °C (lit.) |
| refractive index | 1.500 |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| Specific Gravity | 2.159 |
| Odor | Odorless |
| PH | 8.3 (0.1molfreshly prepared) |
| PH Range | 7.8 - 8.2 |
| pka | (1) 6.37, (2) 10.25 (carbonic (at 25℃) |
| Water Solubility | 9 g/100 mL (20 ºC) |
| Decomposition | 50 °C |
Sodium bicarbonate, used in the formof baking soda and baking powder, is the most common leavening agent. When baking soda,which is an alkaline substance, is added to a mix, it reacts with an acid ingredient to producecarbon dioxide. The reaction can be represented as: NaHCO3(s) + H+ → Na+(aq) + H2O(l) +CO2(g), where H+ is supplied by the acid. Baking powders contain baking soda as a primaryingredient along with acid and other ingredients. Depending on the formulation, bakingpowders can produce carbon dioxide quickly as a single action powder or in stages, as with adouble-action powder. Baking soda is also used as a source of carbon dioxide for carbonatedbeverages and as a buffer.In addition to baking, baking soda has numerous household uses. It is used as a generalcleanser, a deodorizer, an antacid, a fire suppressant, and in personal products such as toothpaste.Sodium bicarbonate is a weak base in aqueous solution, with a pH of about 8. Thebicarbonate ion (HCO3-) has amphoteric properties, which means it can act as either an acidor a base. This gives baking soda a buff ering capacity and the ability to neutralize both acidsand bases. Food odors resulting from acidic or basic compounds can be neutralized with bakingsoda into odor-free salts. Because sodium bicarbonate is a weak base, it has a greater abilityto neutralize acid odors.
The second largest use of sodium bicarbonate, accounting for approximately 25% of totalproduction, is as an agricultural feed supplement. In cattle it helps maintain rumen pH andaids fiber digestibility; for poultry it helps maintain electrolyte balance by providing sodiumin the diet, helps fowl tolerate heat, and improves eggshell quality.
Sodium bicarbonate is used in the chemical industry as a buff ering agent, a blowingagent, a catalyst, and a chemical feedstock. Sodium bicarbonate is used in the leather tanningindustry for pretreating and cleaning hides and to control pH during the tanning process.Heating sodium bicarbonate produces sodium carbonate, which is used for soap and glassmaking.Sodium bicarbonate is incorporated into pharmaceuticals to serve as an antacid, abuff ering agent, and in formulations as a source of carbon dioxide in eff ervescent tablets. Drychemical type BC fire extinguishers contain sodium bicarbonate (or potassium bicarbonate).Other uses of bicarbonate include pulp and paper processing, water treatment, and oil welldrilling.
Sodium Bicarbonate is a leavening agent with a ph of approxi- mately 8.5 in a 1% solution at 25°c. it functions with food grade phosphates (acidic leavening compounds) to release carbon dioxide which expands during the baking process to provide the baked good with increased volume and tender eating qualities. it is also used in dry-mix beverages to obtain carbonation, which results when water is added to the mix containing the sodium bicarbonate and an acid. it is a component of baking powder. it is also termed baking soda, bicarbonate of soda, sodium acid carbonate, and sodium hydrogen carbonate.
manufacture of many sodium salts; source of CO2; ingredient of baking powder, effervescent salts and beverages; in fire extinguishers, cleaning Compounds.
sodium bicarbonate (baking soda) is an inorganic salt used as a buffering agent and a pH adjuster, it also serves as a neutralizer. It is used in skin-smoothing powders.