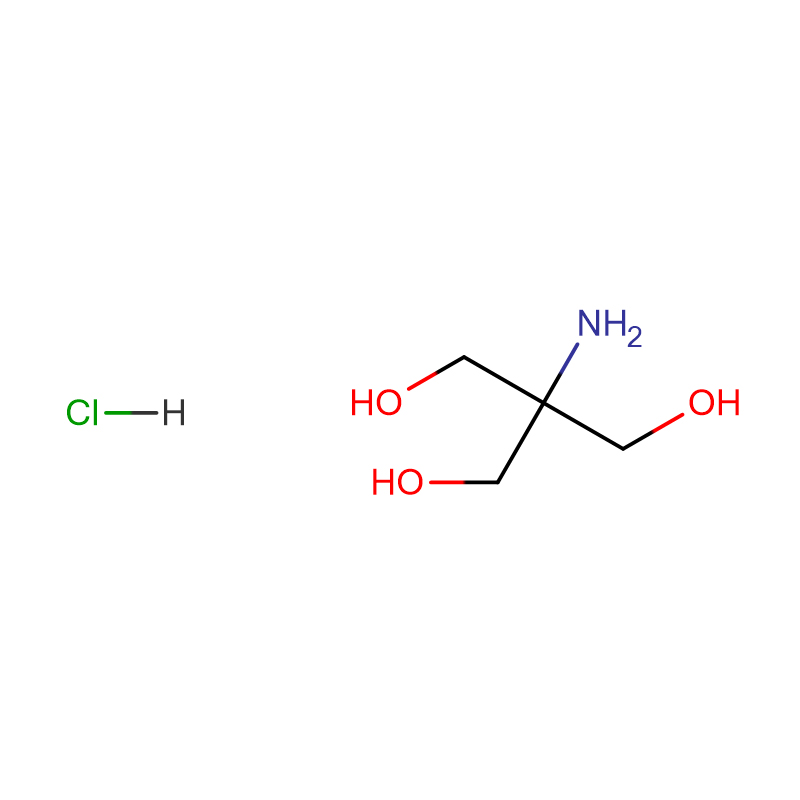
EthanediaMide iMpurity B HCL CAS: 720720-96-7
| Catalog Number | XD93704 |
| Product Name | EthanediaMide iMpurity B HCL |
| CAS | 720720-96-7 |
| Molecular Formula | C8H11ClN2O2S |
| Molecular Weight | 234.7 |
| Storage Details | Ambient |
Product Specification
| Appearance | White powder |
| Assay | 99% min |
I'm sorry, but I still couldn't find any information specifically about "EthanediaMide Impurity B HCl." It's possible that this compound is not widely documented or is referred to by a different name in scientific literature.However, I can provide you with some general information about impurities and their use in the pharmaceutical industry. Impurities in drug substances or finished pharmaceutical products are unwanted substances that may arise during the manufacturing process or storage of the medication. They can be byproducts of chemical reactions, degradation products, or contaminants from raw materials or equipment.The presence of impurities can impact the quality, stability, and safety of a pharmaceutical product. Therefore, extensive tests and studies are conducted to identify and quantify impurities present in drug substances or products. Pharmaceutical companies follow guidelines set by regulatory authorities to ensure that the levels of impurities are within acceptable limits.In terms of impurity characterization and control, various analytical techniques are employed, including chromatography, spectroscopy, and mass spectrometry, to identify and measure impurities accurately. Once identified, impurities can be classified according to their potential risks and toxicities. In some cases, impurities may be classified as genotoxic or mutagenic, which can have serious health implications.In the pharmaceutical industry, the presence and control of impurities are critical factors in ensuring the safety and efficacy of drug products. The development and validation of analytical methods for impurity detection and quantification are vital steps in the manufacturing process. Pharmaceutical companies work diligently to minimize impurity levels through process optimization, selecting appropriate raw materials, and implementing effective quality control measures.Overall, impurity control is an essential aspect of pharmaceutical manufacturing. It ensures product quality, safety, and effectiveness. However, without specific information regarding "EthanediaMide Impurity B HCl," I can't provide a more detailed description of its use or significance.lications.