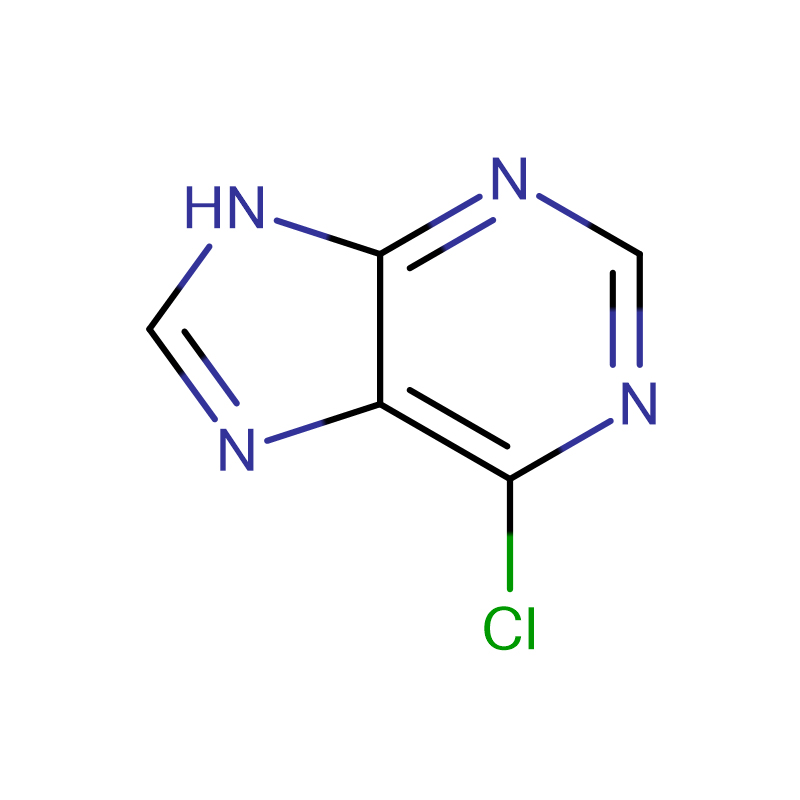
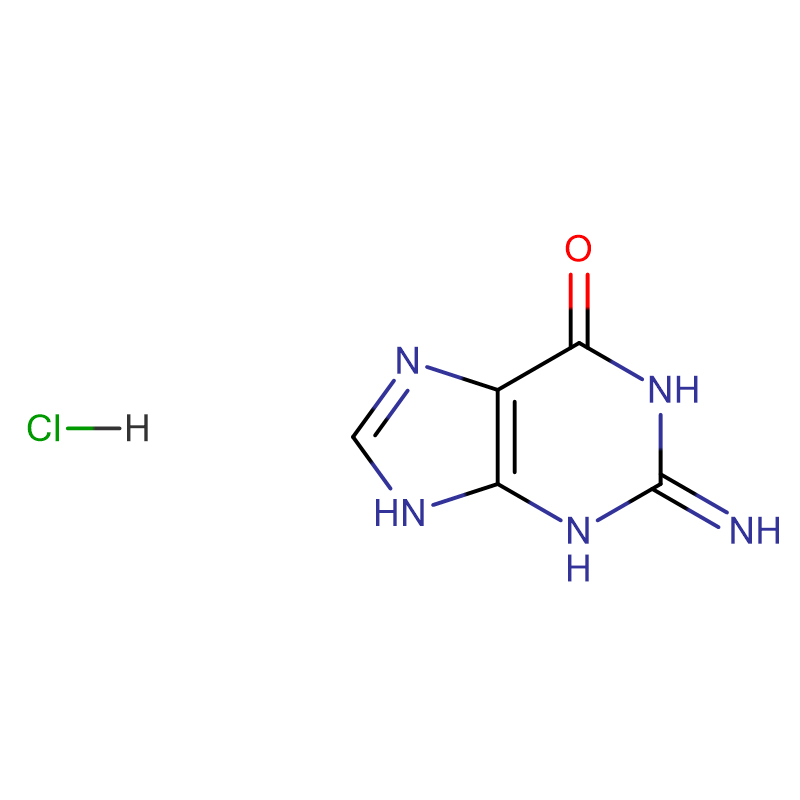
6-Chloropurine CAS:87-42-3 Light yellow powder
| Catalog Number | XD90547 |
| Product Name | 6-Chloropurine |
| CAS | 87-42-3 |
| Molecular Formula | C5H3ClN4 |
| Molecular Weight | 154.557 |
| Storage Details | 2 to 8 °C |
| Harmonized Tariff Code | 2933990090 |
Product Specification
| Appearance | Light yellow powder |
| Assay | 99% |
We have recently reported that pyrene nucleotide is preferentially inserted opposite an abasic site, the 3'-T of a thymine dimer, and most undamaged bases by yeast DNA polymerase eta (pol eta). Because pyrene is a nonpolar molecule with no H-bonding ability, the unusually high efficiencies of dPMP insertion are ascribed to its superior base stacking ability, and underscore the importance of base stacking in the selection of nucleotides by pol eta. To investigate the role of H-bonding and base pair geometry in the selection of nucleotides by pol eta, we determined the insertion efficiencies of the base-modified nucleotides 2,6-diaminopurine, 2-aminopurine, 6-chloropurine, and inosine which would make a different number of H-bonds with the template base depending on base pair geometry. Watson-Crick base pairing appears to play an important role in the selection of nucleotide analogues for insertion opposite C and T as evidenced by the decrease in the relative insertion efficiencies with a decrease in the number of Watson-Crick H-bonds and an increase in the number of donor-donor and acceptor-acceptor interactions. The selectivity of nucleotide insertion is greater opposite the 5'-T than the 3'-T of the thymine dimer, in accord with previous work suggesting that the 5'-T is held more rigidly than the 3'-T. Furthermore, insertion of A opposite both Ts of the dimer appears to be mediated by Watson-Crick base pairing and not by Hoogsteen base pairing based on the almost identical insertion efficiencies of A and 7-deaza-A, the latter of which lacks H-bonding capability at N7. The relative efficiencies for insertion of nucleotides that can form Watson-Crick base pairs parallel those for the Klenow fragment, whereas the Klenow fragment more strongly discriminates against mismatches, in accord with its greater shape selectivity. These results underscore the importance of H-bonding and Watson-Crick base pair geometry in the selection of nucleotides by both pol eta and the Klen ow fragment, and the lesser role of shape selection in insertion by pol eta due to its more open and less constrained active site.