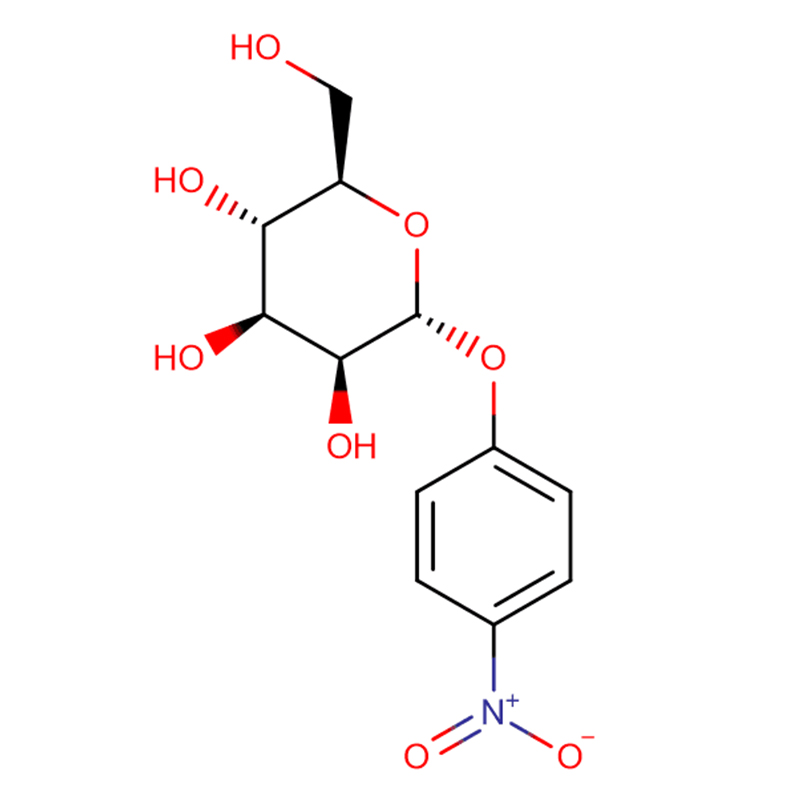
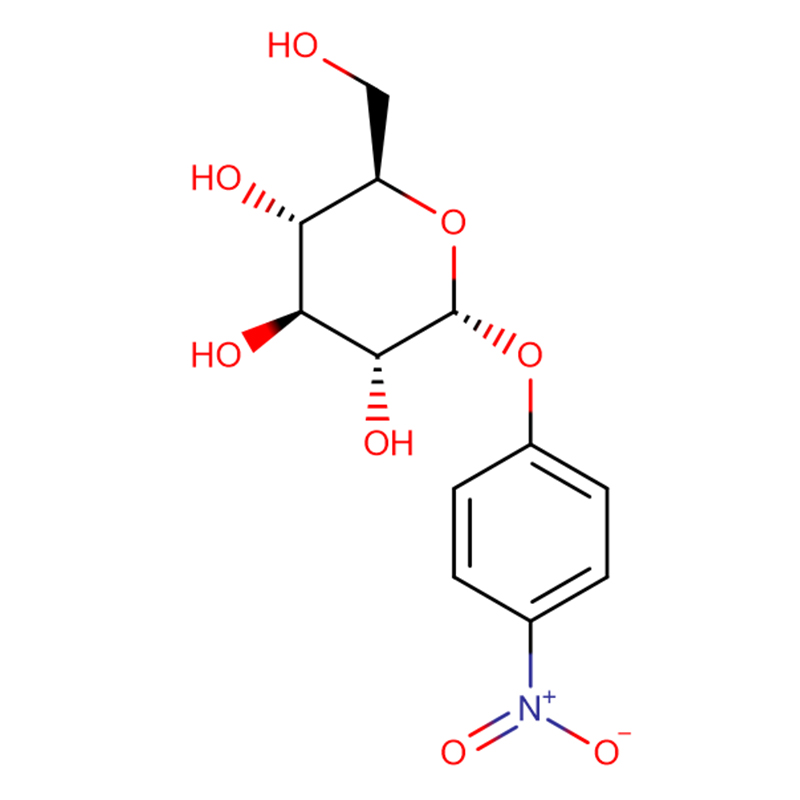
4-NITROPHENYL-ALPHA-D-MANNOPYRANOSIDE CAS:10357-27-4 Off-White Powder 98%
| Catalog Number |
XD90011 |
| Product Name |
4-Nitrophenyl-alpha-D-mannopyranoside |
| CAS |
10357-27-4 |
| Molecular Formula |
C12H15NO8 |
| Molecular Weight |
30 301.25 |
| Storage Details |
-2 to -8 °C |
| Harmonized Tariff Code |
29400000 |
Product Specification
| Water | <5% Karl Fische |
| Solubility | 1% in DMF is clear and colourless |
| Purity | Free 4-Nitrophenol <200ppm |
| HPLC | >98% |
| Appearance | Off-white powder |
Mechanistic insights into a Ca2+-dependent family of alpha-mannosidases in a human gut symbiont.
Colonic bacteria, exemplified by Bacteroides thetaiotaomicron, play a key role in maintaining human health by harnessing large families of glycoside hydrolases (GHs) to exploit dietary polysaccharides and host glycans as nutrients. Such GH family expansion is exemplified by the 23 family GH92 glycosidases encoded by the B. thetaiotaomicron genome. Here we show that these are alpha-mannosidases that act via a single displacement mechanism to utilize host N-glycans. The three-dimensional structure of two GH92 mannosidases defines a family of two-domain proteins in which the catalytic center is located at the domain interface, providing acid (glutamate) and base (aspartate) assistance to hydrolysis in a Ca(2+)-dependent manner. The three-dimensional structures of the GH92s in complex with inhibitors provide insight into the specificity, mechanism and conformational itinerary of catalysis. Ca(2+) plays a key catalytic role in helping distort the mannoside away from its ground-state (4)C(1) chair conformation toward the transition state.(Bibliography: Nat. Chem. Biol. 6, 125-32, (2010)
Frontal affinity chromatography of ovalbumin glycoasparagines on a concanavalin A-sepharose column. A quantitative study of the binding specificity of the lectin.
The interactions of Sepharose 4B-immobilized concanavalin A (ConA) with 10 glycoasparagines derived from ovalbumin were investigated quantitatively by frontal affinity chromatography. In this method, a carbohydrate solution is applied continuously to a ConA-Sepharose column and the retardation of the elution front is measured as a parameter of the strength of the interaction. The dissociation constant (Kd) for each saccharide with ConA can be determined. An analysis of the binding of p-nitrophenyl-alpha,D-mannoside has shown that the binding properties of ConA do not change essentially after immobilization on Sepharose 4B. Each of the ovalbumin glycoasparagines was labeled with tritium by the reductive methylation method for analysis. A comparison of the Kd values obtained showed that the binding of ConA varies considerably with very slight structural differences of the glycosyl chain. The results suggest that ConA recognizes a specific glycosyl chain structure, Man alpha 1-6(Man alpha 1-3)Man, in which at least one hydroxyl group at the C-3 position of C-6-linked mannose should be free.