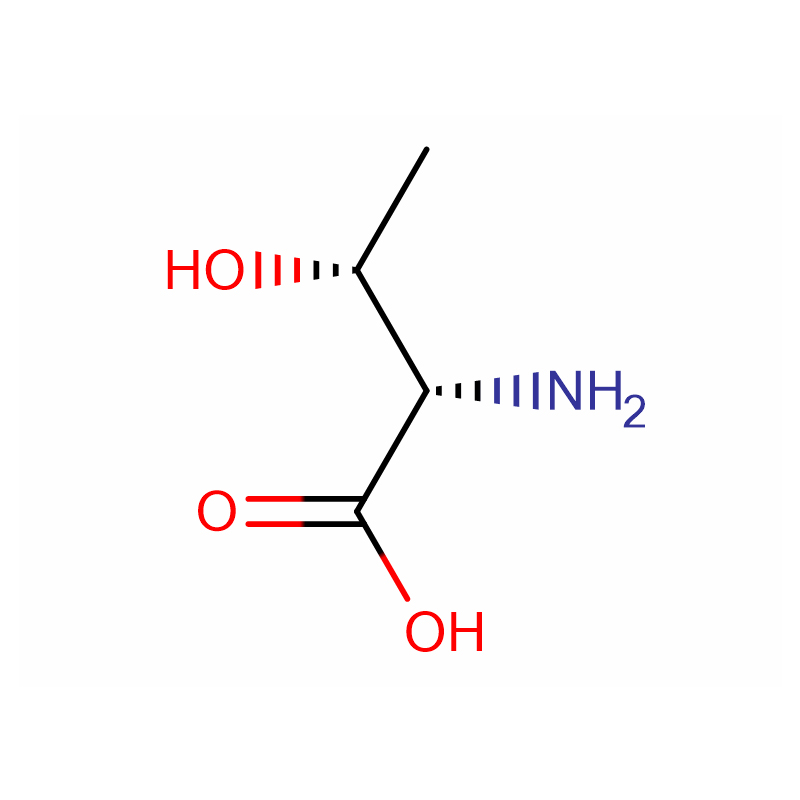
(2S,3R)-2-Amino-3-hydroxybutanoic acid Cas: 72-19-5 99% White crystalline powder
| Catalog Number | XD90285 |
| Product Name | (2S,3R)-2-Amino-3-hydroxybutanoic acid |
|
CAS |
72-19-5 |
|
Molecular Formula |
C4H9NO3 |
|
Molecular Weight |
119.11916 |
| Storage Details | Ambient |
|
Harmonized Tariff Code |
29225000 |
Product Specification
| Assay | 99 - 101% |
| Appearance | White crystalline powder |
| Specific rotation | -27.5 to -29.0 |
| Heavy metals | 10ppm Max. |
| AS | 10ppm max |
| pH | 5.2 - 6.5 |
| SO4 | <0.020% |
| Fe | 10ppm max |
| Loss on Drying | <0.20% |
| Residue on Ignition | <0.10% |
| Transmittance | NLT 98% |
| Cl | <0.02% |
| Ammonium salt | <0.02% |
Serine/threonine protein phosphatases have been described in many pathogenic bacteria as essential enzymes involved in phosphorylation-dependent signal transduction pathways and frequently associated with the virulence of these organisms. An inspection of Mycoplasma synoviae genome revealed the presence of a gene (prpC) encoding a putative protein phosphatase of the protein phosphatase 2C (PP2C) subfamily. Here, we report a complete biochemical characterization of M. synoviae phosphatase (PrpC) and the particular role of metal ions in the structure-function relationship of this enzyme. PrpC amino acid sequence analysis revealed that all the residues involved in the dinuclear metal center and the putative third metal ion-coordinating residues, conserved in PP2C phosphatases, are present in PrpC. PrpC is a monomeric protein able to dephosphorylate phospho-substrates with Mn(2+) ions' dependence. Thermal stability analysis demonstrated the enzyme stability at mild temperatures and the inf luence of Mn(2+) ions in this property. Mass spectrometry analysis suggested that three metal ions bind to PrpC, two of which with an apparent high-affinity constant. Mutational analysis of the putative third metal-coordinating residues, Asp122 and Arg164, revealed that these variants exhibited a weaker binding of manganese ions, and that both mutations affected PrpC phosphatase activity. According to these results, PrpC is a metal-dependent protein phosphatase member with an improved stability in the holo form and with Asp122, possibly implicated in the third metal-binding site, essential to catalytic activity.