p-hydoxybenzoic acid,monosodium Cas:114-63-6 99% White to faint yellow or off-white crystalline powder
| Catalog Number | XD90141 |
| Product Name | p-hydoxybenzoic acid,monosodium |
| CAS | 114-63-6 |
| Molecular Formula | C7H5O3Na |
| Molecular Weight | 160.10 |
| Storage Details | Ambient |
| Harmonized Tariff Code | 2918290000 |
Product Specification
| Appearance | White to faint yellow or off-white crystalline powder |
| Assay | ≥ 99% |
| Density | 1.3750 |
| Melting point | >300 °C(lit.) |
| Boiling point | 336.2°Cat760mmHg |
| Flash point | 171.3°C |
The inhibition of the β-carbonic anhydrases (CAs, EC 4.2.1.1) from the pathogenic fungi Cryptococcus neoformans (Can2) and Candida albicans (Nce103) with a series of 25 branched aliphatic and aromatic carboxylates has been investigated. Human isoforms hCA I and II were also included in the study for comparison. Aliphatic carboxylates were generally millimolar hCA I and II inhibitors and low micromolar/submicromolar β-CA inhibitors. Aromatic carboxylates were micromolar inhibitors of the four enzymes but some of them showed low nanomolar activity against the fungal pathogenic enzymes. 4-Hydroxy- and 4-methoxy-benzoate inhibited Can2 with K(I)s of 9.5-9.9 nM. The methyl esters, hydroxamates, hydrazides and carboxamides of some of these derivatives were also effective inhibitors of the α- and β-CAs were investigated here.
Parabens are among the most frequently used preservatives to inhibit microbial growth and extend the shelf life of a range of consumer products. The objective of the present study was to gain insight into the metabolism of parabens in breast cancer cells (MCF7) since they have demonstrated estrogenic activity towards these cells and have been detected in breast cancer tissues. The toxicity of parabens to MCF7 cells was determined using MTT assays. Hydrolysis of methyl-, butyl and benzyl-paraben to p-hydroxybenzoic acid was analyzed in cultured MCF7 cells and in cellular homogenates. Glucuronidation and sulfoconjugation were studied in MCF7 homogenates, and parabens were analyzed by HPLC. Methyl-paraben was shown to be far less toxic than butyl and benzyl-paraben. Parabens were completely stable in MCF7 homogenates whereas p-nitrophenyl acetate, a substrate type, underwent hydrolysis. MCF7 cell homogenates did not express glucuronidation and sulfoconjugation activities toward parabens. The higher stability of parabens may explain their accumulation in breast cancer tissue.


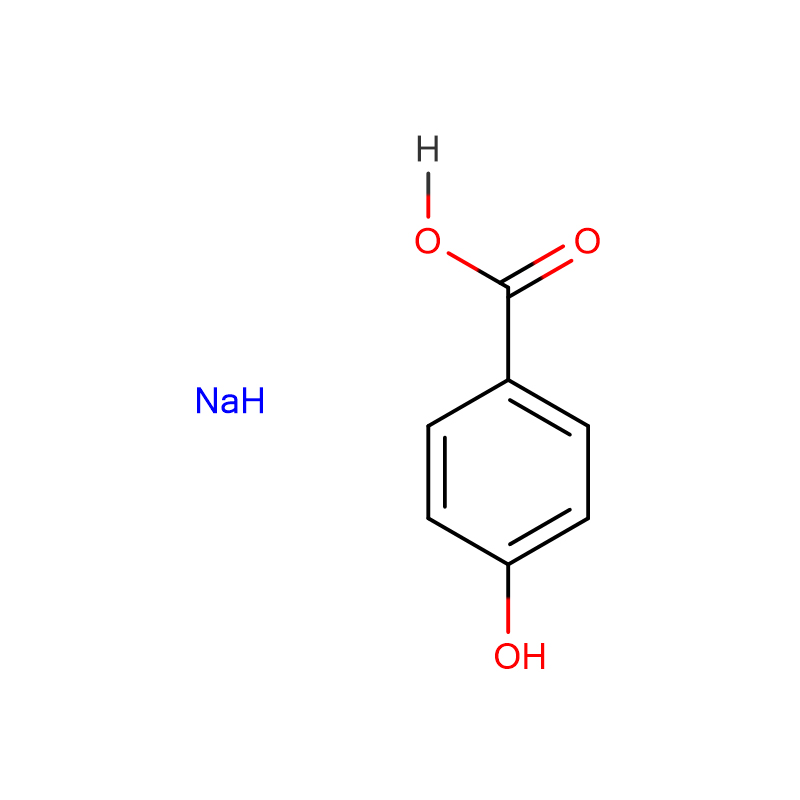

![2-[2-(4-hydroxy-3,5-diiodophenyl)ethenyl]-3,3-dimethyl-1-(3-sulfopropyl)-, inner salt CAS:145876-11-5](https://cdn.globalso.com/xdbiochems/145876-11-5.jpg)